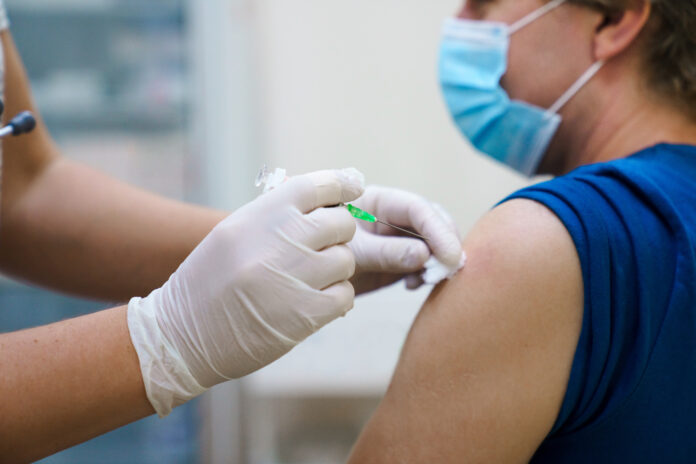
One week after the FDA issued an emergency use authorization (EUA) for the JYNNEOS monkeypox vaccine to allow healthcare providers to inject it intradermally to stretch available resources, Philadelphia is now in a vaccine crisis.
Pennsylvania had expected an allocation of 7,560 vials of vaccine to help contain the rapidly expanding monkeypox outbreak. But now that number has been radically cut, with the state receiving only 1,520 vials, total.
The news scuttles all the plans the Philadelphia Department of Public Health (PDPH) had for expanding access to vaccines for at-risk communities. Health Commissioner Dr. Cheryl Bettigole had previously estimated Philadelphia would require about 24,000 doses of the JYNNEOS vaccine to administer the two shots that are the standard vaccine dosage. Those shots would have inoculated Philadelphians at highest risk of exposure to monkeypox, which has largely impacted gay and bisexual men and men who have sex with men, with men who have HIV at highest risk.
On August 16, PGN received a statement from the PDPH “in response to an announcement by the U.S. Department of Health and Human Services’ Administration for Strategic Preparedness and Response (ASPR) about changes to the City’s monkeypox vaccine allocation.”
The number of vaccines allotted to Philadelphia, whose metro population is 5.7 million, has been slashed to less than a fifth of what was expected for the city. Philadelphia is also getting nearly half the vaccine allotment for the entire state.
Bettigole said that late Monday night “the Administration for Strategic Preparedness and Response [which is the sole distributor of the JYNNEOS vaccine] announced that Philadelphia’s allocation for monkeypox vaccine has been cut following the announcement of a new dose conserving strategy. Previously, the City expected to receive 3,612 vials, but has been told that only 720 vials will be allocated to Philadelphia.”
Bettigole said, “The dose conserving strategy hinges on a plan to inject one-fifth of a normal dose of vaccine into the layers of the skin rather than the traditional dosing into the subcutaneous area under the skin.”
As PGN has reported, demand for monkeypox vaccines has vastly outstripped available supply. The prescribed two-shot dosage is largely unavailable throughout hard-hit areas, as healthcare administrators have been rationing the vaccine to one shot to allow for more people to receive at least one vaccination and thus be partially protected.
As Dr. Michael LeVasseur, an epidemiologist and public health specialist focused on HIV and health disparities among sexual and gender minority populations at Drexel’s Dornsife School of Public Health, told local media, “Getting a vaccine appointment is like hitting the lottery. It’s like the ‘Hunger Games’.”
The FDA had been tracking this encroaching crisis for weeks prior to the decision to alter the vaccine injection process. “In recent weeks the monkeypox virus has continued to spread at a rate that has made it clear our current vaccine supply will not meet the current demand,” said FDA Commissioner Robert M. Califf, M.D. “The FDA quickly explored other scientifically appropriate options to facilitate access to the vaccine for all impacted individuals. By increasing the number of available doses, more individuals who want to be vaccinated against monkeypox will now have the opportunity to do so.”
Bettigole’s statement attempted to clarify the new FDA strategy, something she had said she would be exploring the day after the FDA emergency authorization was announced.
Bettigole now explains that “since the U.S. FDA’s announcement of the dose conserving strategy, the Health Department has been reviewing the scientific literature, speaking with experts, and reaching out to vaccine providers and community members to understand their perspective on the new recommendation prior to making a decision on the new approach.”
But plans the PDPH had for a more widely expanded vaccine program from the invitation-only plan currently in place have been completely altered by news of the federal cuts to supply. The PDPH has already been under fire from community organizations like Black and Latinx Community Control of Health (BLCC) and ACT UP Philadelphia over who is being prioritized for vaccines.
The PDPH had announced August 11 that sex workers in Philadelphia were now eligible to receive the monkeypox vaccine. PDPH spokesperson James Kyle said that the addition of sex workers to the list of those eligible for the vaccine was because they are at high risk of contracting the virus.
“At this point, we haven’t seen cases among healthcare workers or unhoused individuals who don’t also have other risk factors,” Kyle told local media. “However, these are important groups to watch, and we hope to be able to vaccinate these groups as soon as we have enough vaccine.”
Those plans are now on hold.
As Bettigole explained, “We had hoped to announce that, due to the ability to quintuple the number of doses available in Philadelphia for those that this strategy is indicated for, we would be widely expanding eligibility and could work to protect people who might be exposed.”
She said, “This is different than our current strategy of focusing our limited doses on only those who have already been exposed. By getting ahead of the virus’s spread, we could actually prevent further spread of this virus, which causes painful lesions that can last up to 4 weeks and may leave permanent scars.”
But there are complications in administering the intradermal injections, including that they require smaller needles and precision in pricking the skin to deliver the dosage.
Bettigole acknowledged that “the dose conserving strategy is not easily adopted. There are logistical issues with changing how doses are tracked, issues with ensuring that staff are trained on the intradermal injection technique, and issues with securing the smaller syringes required for this type of vaccination.”
She added, “There are also issues with who is indicated to receive this vaccine in this manner, as those who have a history of keloid formation should not receive their doses intradermally. Even with all of those issues, we were confident that we could vaccinate more people by integrating this strategy into our response.”
Black people are especially prone to keloid formation, which puts them at risk from the new dosing technique.
Trying to work within the latest setback puts additional stress on an already overworked health department and local healthcare providers caring for at-risk groups. Bettigole said that after this announcement, the PDPH “will need to review plans and try to figure out how to make the most of this situation. We are advocating to our federal partners to reconsider and restore Philadelphia’s allocation of vaccine, which is urgently needed. At the same time, we have no choice but to ask all partners to convert to using the intradermal strategy for all eligible patients.”
She said that it was essential to shift to “using 1/5th of the conventional dose, as soon as possible and to conserve vaccine for only those with an urgent need due to a known or suspected exposure until they are able to make that change.”
Noting that “this is far from ideal in the midst of an outbreak,” Bettigole admitted that “with no guarantee of additional doses before September, we see no other choice.”
PDPH reports that the total number of doses administered as of August 16 is 3,877, the number of doses received to date in Philadelphia is 6,445, the doses given to providers is 1,530, and the remaining doses available (until next delivery, expected to be September or later, when the 720 will be delivered) is 1,038.