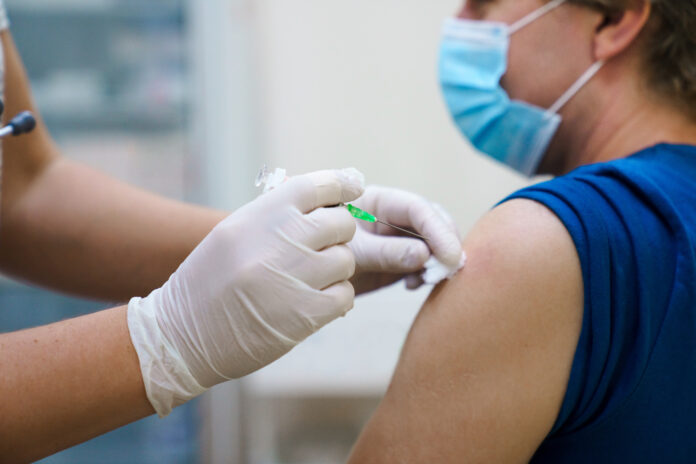
As monkeypox cases continue to rise in the U.S., with 9,492 cases as of August 9, the Food and Drug Administration (FDA), in concert with the Centers for Disease Control and Prevention (CDC), has devised a plan to extend the limited supply of JYNNEOS monkeypox vaccine by cutting the dosage of what is currently prescribed.
As PGN previously reported, demand for monkeypox vaccines has vastly outstripped available supply. The prescribed two-shot dosage is unavailable almost everywhere, as health care administrators have been rationing the vaccine to one shot to allow for more people to receive at least one vaccine and thus be somewhat protected.
While the Biden administration has asserted that vaccines will be available, the timeline for those vaccines is muted: the next delivery won’t be until October at the earliest and most of the vaccines bought by the administration won’t be in the U.S. until May 2023 — nearly a year from now.
With a looming crisis of widespread contagion, the FDA announced on August 9 that rather than the prescribed two-shot dosage, the agency has issued an emergency use authorization (EUA) for the JYNNEOS vaccine to allow healthcare providers to use the vaccine by intradermal injection for individuals 18 years of age and older who are determined to be at high risk for monkeypox infection.
“In recent weeks the monkeypox virus has continued to spread at a rate that has made it clear our current vaccine supply will not meet the current demand,” said FDA Commissioner Robert M. Califf, M.D. “The FDA quickly explored other scientifically appropriate options to facilitate access to the vaccine for all impacted individuals. By increasing the number of available doses, more individuals who want to be vaccinated against monkeypox will now have the opportunity to do so.”
This new method of delivery will increase the total number of doses available for use to five times the current availability of the scarce vaccine: one shot will be turned into five. The amount of vaccine previously used to give one person one dose will now be divided between five people.
Two doses of the vaccine given 28 days apart will still be needed for complete protection. But the FDA states there is no data available to indicate that one dose of JYNNEOS will provide long-lasting protection, which will be needed to control the current monkeypox outbreak.
The FDA cites data from a 2015 clinical study of the JYNNEOS vaccine which evaluated a two-dose series given intradermally compared to subcutaneously. The FDA notes, “Individuals who received the vaccine intradermally received a lower volume (one fifth) than individuals who received the vaccine subcutaneously. The results of this study demonstrated that intradermal administration produced a similar immune response to subcutaneous administration, meaning individuals in both groups responded to vaccination in a similar way.”
The FDA also explained the side-effects from both forms of delivery, noting, “Administration by the intradermal route resulted in more redness, firmness, itchiness and swelling at the injection site, but less pain, and these side effects were manageable. The FDA has determined that the known and potential benefits of JYNNEOS outweigh the known and potential risks for the authorized uses.”
The FDA EUA will also allow use of the vaccine in children determined to be at high risk of monkeypox infection, like those exposed in a recent daycare setting in Illinois. For these children the vaccine will be given via the standard two-dose subcutaneous injection.
In Pennsylvania, the expanding case load mirrors much of the country, though cases are still considered vastly under-counted both in the state and nationwide. In less than a month Pennsylvania went from 27 confirmed cases of monkeypox to 251 as of August 9. Pennsylvania now has the 7th most cases in the country, according to CDC mapping. The most cases are in New York, California, Texas and Illinois.
Since May, when the first case was confirmed in Massachusetts, the U.S. caseload has increased from 866 cases on July 11 to the current 9,494 only a month later. Wyoming is the only state with no reported cases.
PGN asked the Philadelphia Department of Public Health (PDPH) about the current state of the virus in Philadelphia and the change in protocols. The PDPH issued a statement from Health Commissioner Dr. Cheryl Bettigole.
“The Health Department is grateful to the federal government for exploring options to expand the availability of monkeypox vaccine,” said Bettigole, who said the FDA announcement “highlights the difficult situation Philadelphia is in, with many more people needing vaccine than is available.”
She also raised the question of whether this new plan was optional for patients or the only choice. Bettigole said, “We look forward to learning more about the federal government’s plan, including additional information on the safety and efficacy of the proposed dosing strategy, as well as clarity on whether jurisdictions and/or vaccine providers and patients have the option of choosing between the proposed intradermal dosing strategy and the usual subcutaneous injection route or whether the new intradermal strategy will be the only available option.”
The Commissioner added, “While we continue to work to ensure that as many at risk Philadelphians are protected as possible while we wait for those answers, the Health Department is beginning to plan for implementation of this new vaccine strategy, if it’s required.”
PGN asked if the PDPH was doing anything in particular to ramp up testing and/or access to anti-virals like TPOXX/tecovirimat, which men nationwide have complained is as hard to access as the vaccine.
Matthew Rankin, Media Coordinator for PDPH, told PGN, “If a resident has symptoms of monkeypox, including a rash or lesions, they should call their regular healthcare provider immediately. Health providers are the best way for residents to get tested for the virus if they feel they have been exposed.”
Rankin explained that PDPH “has started to work with Low-Threshold Sexual Health Sites, our Health Centers, and other public clinics to provide resources and/or training for specimen collection and submission of specimens for testing.”
He also said “The Health Department continues to work with healthcare providers in Philadelphia to administer TPOXX when indicated. The Health Department has been sharing information with healthcare providers about testing and treatment for Monkeypox as guidance.”
PGN asked if there was outreach specifically to communities of color, as Philadelphia is more than 70 percent people of color and there have been concerns that the vaccine is being distributed largely to white residents.
Rankin said, “The Health Department continues to reach communities of color through tabling events with organizations city wide. Staffed informational events are upwards of 30 events a week all over the city.”
He added that the “Health Department staff have been holding one on one meetings with specific organizations like FIGHT and Philly Black Pride who have offered feedback allowing us to provide up to date information at targeted locations. The Health Department continues to identify additional organizations to listen to concerns and receive feedback on how to best serve our communities.”
PGN asked the Mazzoni Center, which has been the city’s main outlet for testing and vaccinations, if Mazzoni was considering using the ACAM2000 smallpox vaccine, which the CDC has cited as another available vaccine for monkeypox, or if that vaccine, which is live, was contraindicated by the demographic of clients served by the Center?
Larry Benjamin, Director of Communications at Mazzoni, told PGN “Currently, we have JYNNEOS as that is what is available from the City. If ACAM2000 becomes available, we would administer it to those patients who can receive it. The vaccines have differing patient populations and side effects to be considered individually.”
When asked about the new FDA vaccine protocols, Benjamin told PGN,
“We’re glad the FDA is hearing the urgent calls from not only organizations here in Philadelphia, but from people all across the country that we need more access to vaccines. This change is definitely a positive step in ensuring that we can vaccinate as many individuals as possible.”
He added, “We know the Philadelphia Department of Public Health is working on taking the guidance that was issued and rolling that out to health care providers like us, Bebashi, and others who are distributing the vaccine.”
Benjamin said that through August 8, Mazzoni has “received 600 doses and administered 325 doses of JYNNEOS monkeypox vaccine. While our priority has been vaccinating as many of our patients who meet the vaccine criteria as possible, we have also been very intentional to ensure our distribution is equitable.”
He explained that “the majority of doses have been administered to folks between 25 and 64, 40% to folks of color or who do not identify any race with us, and reflective of our insurance demographics including 11% for people without insurance.”
With students heading back to school on August 29, PGN asked PDPH if there were any alerts for daycares and schools, to which Rankin said, “We know that this virus spreads in a couple specific ways. Through direct skin-to-skin contact or prolonged face-to-face interaction. It is not impossible, but very unlikely that you would see spread in a school settings. Currently the monkeypox virus seems to have been limited to specific social networks.”
He added, “At this time, we have not seen advancement of spread to the city at large. There are certain settings we will watch closely including sports with close contact, like wrestling, and in early childhood settings where there is more hands-on care.”
The PDPH told PGN on August 3 that “5,045 is the total number of doses we’ve received. Also, we expect to be able to order more on August 15.”
Though unable to confirm the timeline exactly, the spokesperson said that, as in other cities, the expected receipt of new doses would be October. A spokesperson told PGN, “Until we receive a supply of vaccine sufficient to inoculate everyone who wants it, we will continue to prioritize those most at risk.”
For info on monkeypox in Philadelphia, visit https://www.phila.gov/. For CDC data on and symptoms and prevention of monkeypox, visit https://www.cdc.gov.