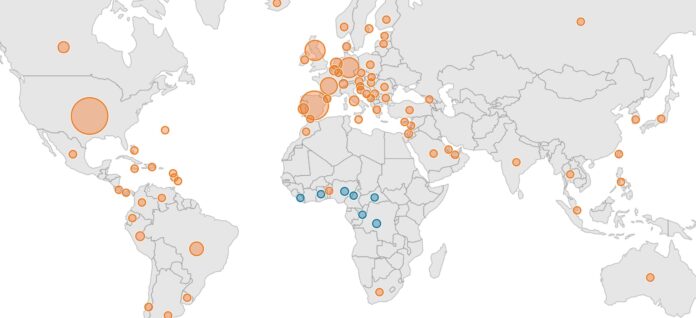
The U.S. now leads the world in monkeypox cases as the virus continues to spread rapidly and globally. There are more than 25,000 cases worldwide in 83 countries; in only seven of those countries is monkeypox endemic. Thus far there are more than 6,300 confirmed cases in the U.S., with 48 states, plus D.C. and Puerto Rico, reporting cases. That number is nearly double what it was a week ago.
But testing remains limited, suggesting the caseload is far greater both in the U.S. and elsewhere. New York, California, Illinois — sites of the nation’s three largest cities — have reported nearly half of all confirmed monkeypox infections in the U.S. and some cities, like New York, San Francisco and Los Angeles, have declared states of emergency. Philadelphia has not followed suit, but Pennsylvania’s reported case count of only 170 cases as of August 2 seems vastly under-reported, given both the proximity to New York and the population of Philadelphia.
The state and city declarations follow the World Health Organization’s own announcement of a global health emergency which many experts cite as far later than demanded by the scope of the outbreak.
Despite President Biden himself raising alarms over the monkeypox outbreak back in May, the White House did not announce a team to lead the monkeypox response until August 2.
Why did it take the Biden administration three months to designate a monkeypox response team? Why does testing remain inadequate? And most critically — where are the Jynneos monkeypox vaccines to contain and control the outbreak in the U.S.?
Biden chose FEMA’s Robert Fenton to be the White House National Monkeypox Response Coordinator. Dr. Demetre Daskalakis will be the White House National Monkeypox Response Deputy Coordinator. Fenton previously coordinated testing and vaccines for COVID-19 for the Biden administration. Daskalakis, who is openly gay, has a long history in New York of working with gay, bisexual and men who have sex with men on sexual health issues, including HIV/AIDS.
Fenton and Daskalakis “will lead the administration’s strategy and operations to combat the current monkeypox outbreak, including equitably increasing the availability of tests, vaccinations and treatments.”
Activists in the LGBTQ community as well as experts on monkeypox have cited the CDC’s (Centers for Disease Control and Prevention) slow and confusing response to the outbreak as allowing both stigma to adhere to the gay and bisexual community and also response for prophylaxis to lag.
Demand for monkeypox vaccines has vastly outstripped available supply as infections continue to rise. New York Mayor Adams has challenged HHS on the lack of availability as New York has more than a third of all U.S. cases. Staff at sexual health clinics and other sites have been unable to serve the influx of men seeking the shots and nearly everywhere the two-shot dosage is completely unavailable, with health care administrators rationing the vaccine to one shot to allow for more people to receive at least one vaccine and thus be somewhat protected.
The failure to vaccinate early is perceived by LGBTQ activists as a failure to address the demographic currently most at risk for the disease: gay and bisexual men and men who have sex with men. The conflation of monkeypox into an STI has added both stigma and a lapse in treatment and prophylactic protocols from local health departments as well as the CDC. CDC Director Dr. Rochelle Walensky, whose problematic response to COVID-19 has caused many in the disability community to call for her resignation, has been dismissive of the impact of the outbreak.
Dr. Scott Gottlieb, former U.S. Food and Drug Administration (FDA) Commissioner, has said that it may be too late to control and contain the virus and that the U.S. and CDC have made “a lot of the same mistakes” by failing on testing and lack of vaccines distributed to the communities most impacted.
In an essay for the New York Times on July 30, Gottlieb, who has been excoriating the CDC on their slow response to monkeypox for weeks, noted that early reports in May of community spread outside the African countries where monkeypox is endemic “should have been a code red for federal infectious disease response.”
Gottlieb noted, “But it wasn’t until late June that the Centers for Disease Control and Prevention expanded testing for monkeypox to large commercial labs like Quest Diagnostics and Labcorp for more capacity and access. The CDC had gone through its standard playbook, ticking through its protracted checklist.”
The result has been catastrophic, as the doubling of cases in a week has indicated.
Added to this failure to respond quickly and appropriately, is the lack of vaccine availability. While the Biden administration has touted that vaccines will be available, the timeline for those vaccines is in the fine print: Most won’t be in the U.S. until May 2023 — nearly a year from now. What’s more, in 2014, during the Obama administration, there were 20 million vaccines stockpiled — an adequate number to stave off the current outbreak — as detailed in the HHS “2014 Public Health Emergency Medical Countermeasures Enterprise (PHEMCE) Strategy and Implementation Plan.”
But those vaccines were, inexplicably, allowed to expire, while the FDA worked on a freeze-dried vaccine. But that has not happened. Instead raw vaccine, not yet in vials, sits in a Danish warehouse of the biotech company Bavarian Nordic, which developed Jynneos and is sole producer of the vaccine in the world.
The U.S. has subsidized Bavarian Nordic for over a decade and did receive those 20 million doses of Jynneos. But now the U.S. is faced with the lack of vaccines and the inability to address the exponential explosion in cases.
PGN requested comment from HHS and was told by a spokesperson, “We were required to have vaccines for smallpox, based on a material threat determination that simply didn’t exist for monkeypox, specifically.”
The spokesperson said in their statement, “Today, now that we’re in response mode of facing a public health threat, we’re working swiftly around the clock to accelerate the number of doses available.”
So far, the United States has purchased 7 million doses of Bavarian Nordic’s Jynneos vaccine, the preferred vaccine against monkeypox, and the entire supply is expected to be available by mid-2023. Last week, FDA announced it had cleared an additional 786,000 vaccine doses for use. More vaccine is not expected until October.
The Philadelphia Health Department told PGN on August 3 that “5,045 is the total number of doses we’ve received. Also, we expect to be able to order more on August 15.”
Though unable to confirm the timeline exactly, the health department spokesperson said that, as in other cities, the expected receipt of new doses would be October.
A spokesperson told PGN, “Until we receive a supply of vaccine sufficient to inoculate everyone who wants it, we will continue to prioritize those most at risk.”
But is that even possible with only 5,000 doses of vaccine allocated for a city with a metro area of 5.7 million?
As the Washington Post reported July 30, the U.S. faces a “vaccine cliff” on monkeypox, noting “as many as 1 million high-risk men may be unable to get two Jynneos doses for months.”
No more Jynneos vaccines are scheduled to arrive until October at the earliest. According to experts, 3.2 million doses would be necessary to fully cover the at-risk populations designated by the CDC as needing to receive vaccinations. But that amount of vaccine would not be available until mid-2023.
HHS declined to comment on PGN’s request for an answer on whether the administration would be utilizing the more complicated and dangerous smallpox vaccine, ACAM2000, which is not approved for monkeypox.
Routine vaccination of the American public against smallpox stopped in 1972 after the disease was eradicated in the U.S. There is conflicting information on whether people who were vaccinated for smallpox before 1972 are protected against monkeypox.
